`color{green}(text(Structural Variability ))` : The actinoid metals are all silvery in appearance but display a variety of structures.
● The structural variability is obtained due to irregularities in metallic radii which are far greater than in lanthanoids.
`color{green}(text(Reactivity ))` : The actinoids are highly reactive metals, especially when finely divided.
● Example : The action of boiling water on them gives a mixture of oxide and hydride and combination with most non metals takes place at moderate temperatures.
● Hydrochloric acid attacks all metals but most are slightly affected by nitric acid owing to the formation of protective oxide layers; alkalies have no action.
`color{green}(text(Magnetic Properties ))` : The magnetic properties of the actinoids are more complex than those of the lanthanoids. Although the variation in the magnetic susceptibility of the actinoids with the number of unpaired `color{red}(5 f)` electrons is roughly parallel to the corresponding results for the lanthanoids, the latter have higher values.
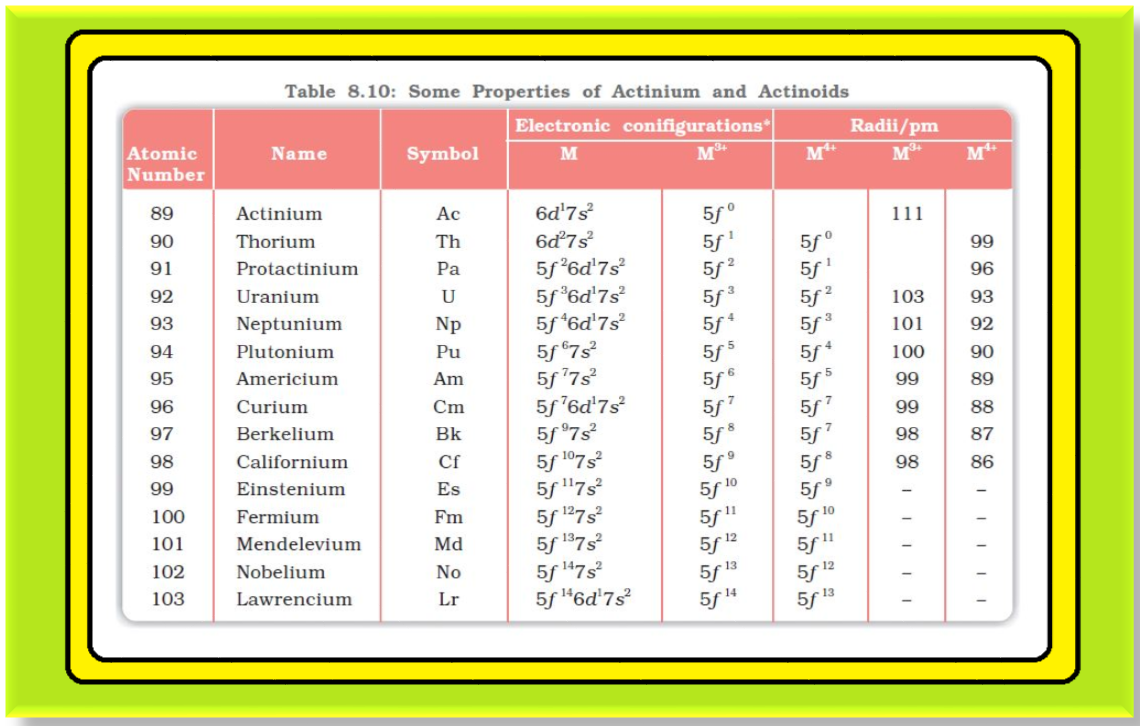
`color{green}(text(Ionisation Enthalpy ))` : It is evident from the behaviour of the actinoids that the ionisation enthalpies of the early actinoids, though not accurately known, but are lower than for the early lanthanoids.
● This is quite reasonable since it is to be expected that when `color{red}(5f)` orbitals are beginning to be occupied, they will penetrate less into the inner core of electrons.
● The `color{red}(5f)` electrons, will therefore, be more effectively shielded from the nuclear charge than the `color{red}(4f)` electrons of the corresponding lanthanoids.
● Because the outer electrons are less firmly held, they are available for bonding in the actinoids.
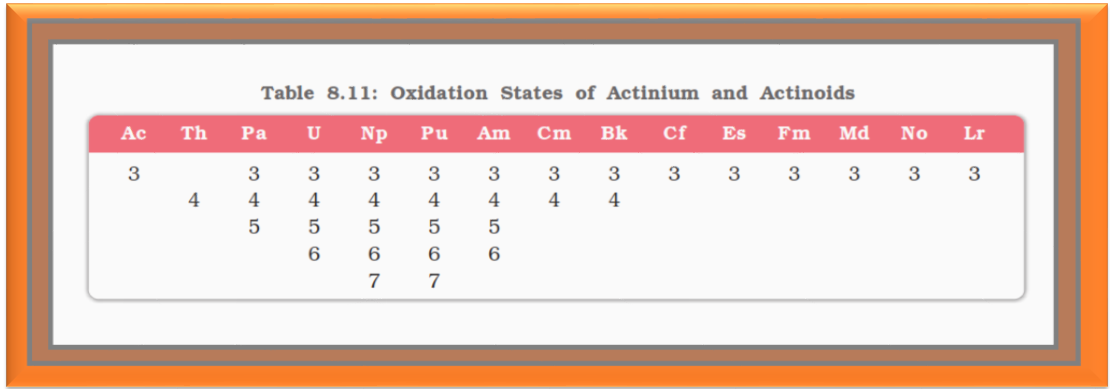
`=>`A comparison of the actinoids with the lanthanoids reveals that behaviour similar to that of the lanthanoids is not evident until the second half of the actinoid series.
`=>` However, even the early actinoids resemble the lanthanoids in showing close similarities with each other and in gradual variation in properties which do not entail change in oxidation state.
`=>` The lanthanoid and actinoid contractions, have extended effects on the sizes, and therefore, the properties of the elements succeeding them in their respective periods.
`=>` The lanthanoid contraction is more important because the chemistry of elements succeeding the actinoids are much less known at the present time.
`color{green}(text(Structural Variability ))` : The actinoid metals are all silvery in appearance but display a variety of structures.
● The structural variability is obtained due to irregularities in metallic radii which are far greater than in lanthanoids.
`color{green}(text(Reactivity ))` : The actinoids are highly reactive metals, especially when finely divided.
● Example : The action of boiling water on them gives a mixture of oxide and hydride and combination with most non metals takes place at moderate temperatures.
● Hydrochloric acid attacks all metals but most are slightly affected by nitric acid owing to the formation of protective oxide layers; alkalies have no action.
`color{green}(text(Magnetic Properties ))` : The magnetic properties of the actinoids are more complex than those of the lanthanoids. Although the variation in the magnetic susceptibility of the actinoids with the number of unpaired `color{red}(5 f)` electrons is roughly parallel to the corresponding results for the lanthanoids, the latter have higher values.
`color{green}(text(Ionisation Enthalpy ))` : It is evident from the behaviour of the actinoids that the ionisation enthalpies of the early actinoids, though not accurately known, but are lower than for the early lanthanoids.
● This is quite reasonable since it is to be expected that when `color{red}(5f)` orbitals are beginning to be occupied, they will penetrate less into the inner core of electrons.
● The `color{red}(5f)` electrons, will therefore, be more effectively shielded from the nuclear charge than the `color{red}(4f)` electrons of the corresponding lanthanoids.
● Because the outer electrons are less firmly held, they are available for bonding in the actinoids.
`=>`A comparison of the actinoids with the lanthanoids reveals that behaviour similar to that of the lanthanoids is not evident until the second half of the actinoid series.
`=>` However, even the early actinoids resemble the lanthanoids in showing close similarities with each other and in gradual variation in properties which do not entail change in oxidation state.
`=>` The lanthanoid and actinoid contractions, have extended effects on the sizes, and therefore, the properties of the elements succeeding them in their respective periods.
`=>` The lanthanoid contraction is more important because the chemistry of elements succeeding the actinoids are much less known at the present time.